ENLIVEN trial
/
Pooled analysis

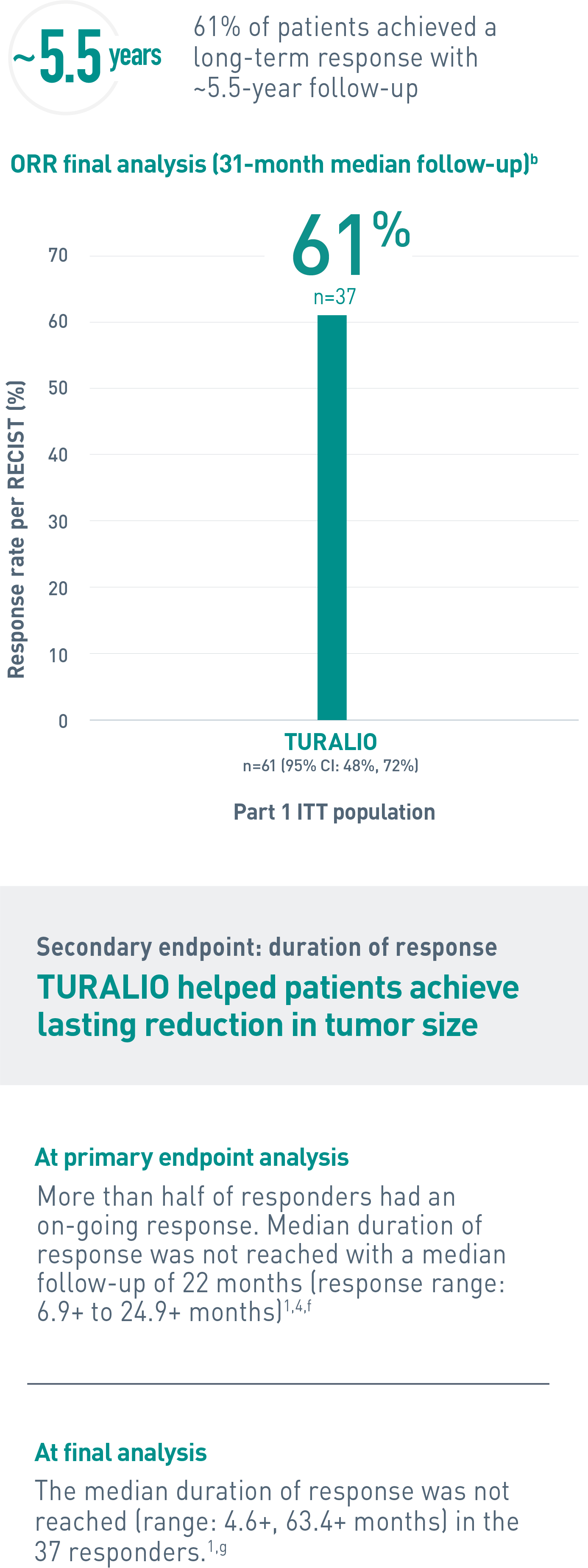
Duration of response is defined as the time when the patient experiences first response to the time of progression, regardless of whether the patient has discontinued treatment.4
CI, confidence interval; ITT, intent-to-treat; ORR, overall response rate; RECIST, Response Evaluation Criteria In Solid Tumors.
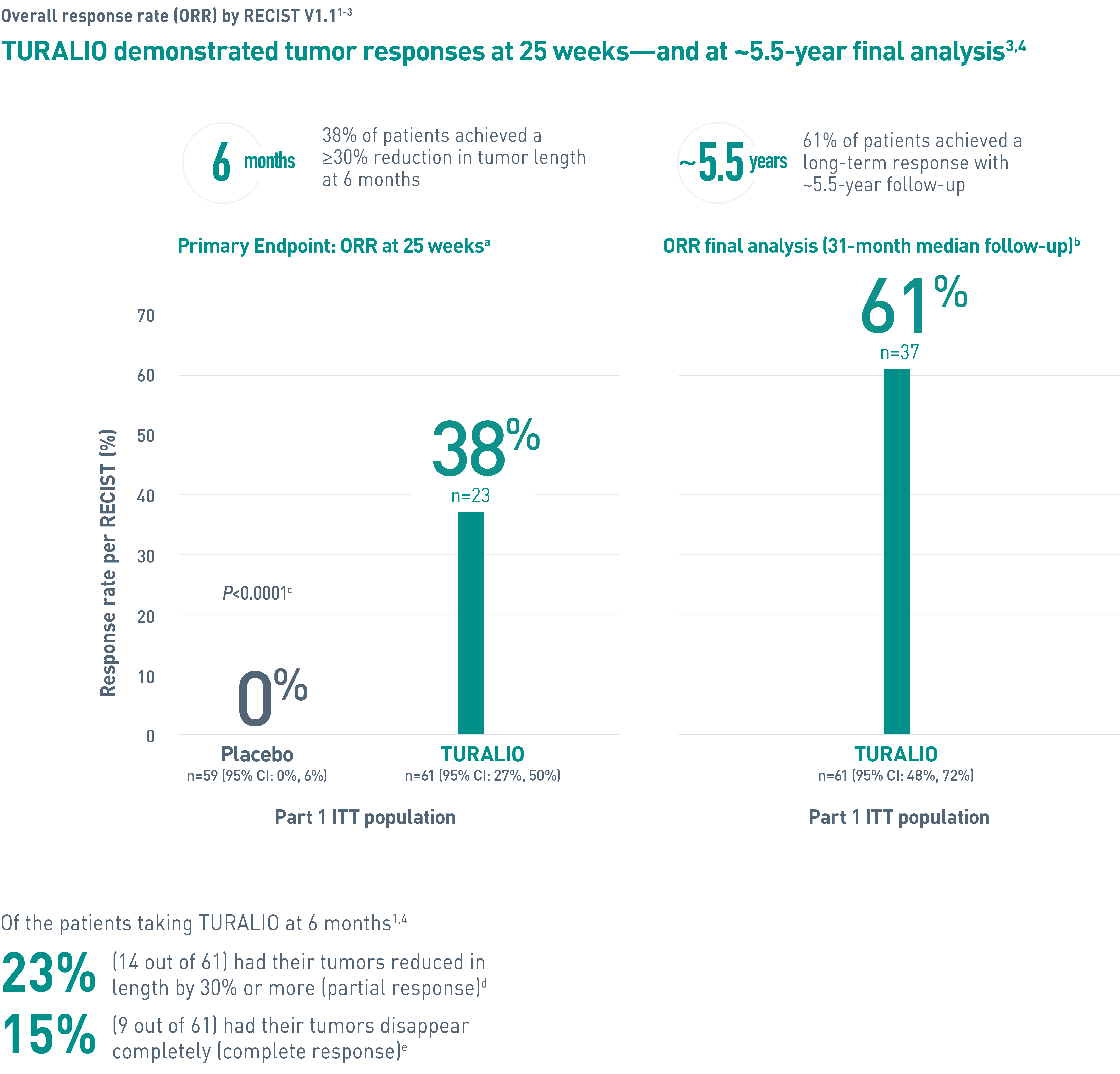
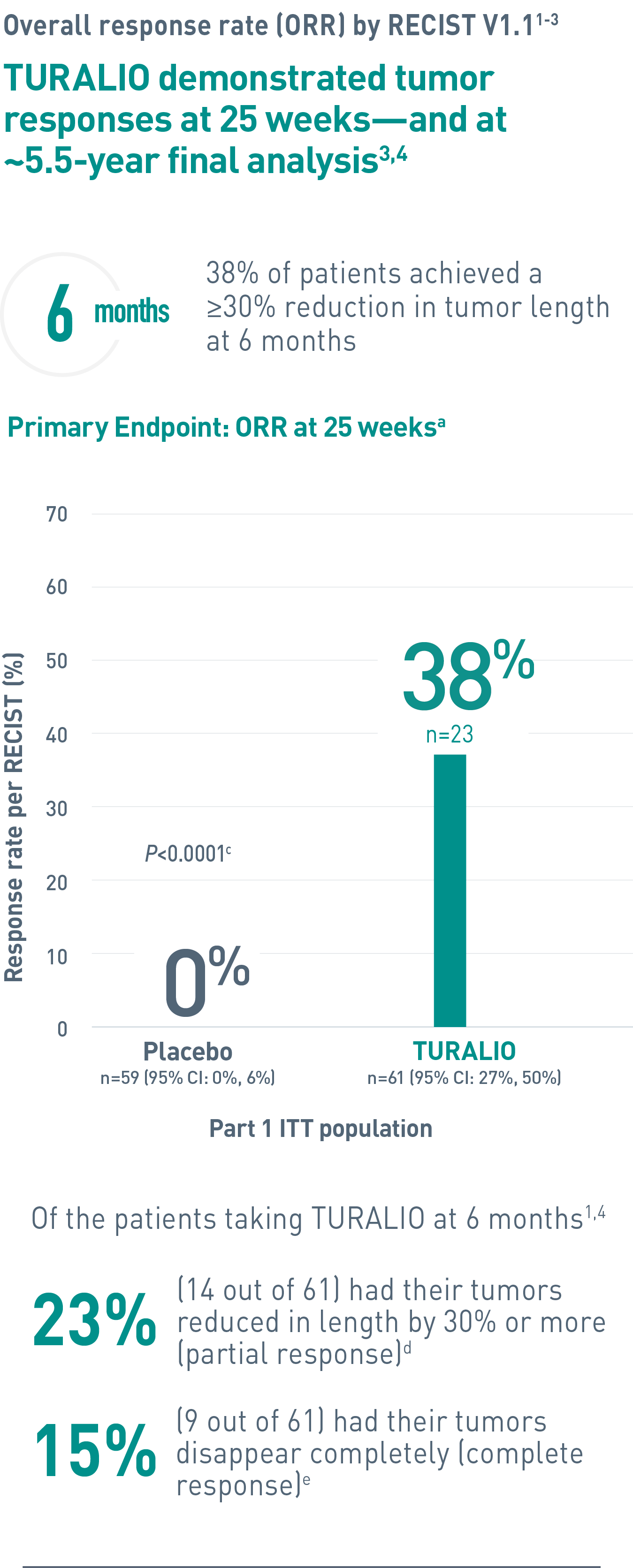
aORR was determined by blinded independent central review (BICR).1
bA median follow-up of 31.2 (range: 2, 66) months (final database lock June 1, 2021).3
cFisher’s exact test.4
dPartial response was defined as 30% or more reduction in tumor length.2
eComplete response was defined as 100% reduction in tumor length.2
fData cutoff January 31, 2018.1
gAt completion of the ENLIVEN study.1
Duration of response is defined as the time when the patient experiences first response to the time of progression, regardless of whether the patient has discontinued treatment.4
CI, confidence interval; ITT, intent-to-treat; ORR, overall response rate; RECIST, Response Evaluation Criteria In Solid Tumors.
aORR was determined by blinded independent central review (BICR).1
bA median follow-up of 31.2 (range: 2, 66) months (final database lock June 1, 2021).3
cFisher’s exact test.4
dPartial response was defined as 30% or more reduction in tumor length.2
eComplete response was defined as 100% reduction in tumor length.2
fData cutoff January 31, 2018.1
gAt completion of the ENLIVEN study.1
 TURALIO is the first FDA-approved targeted therapy for adults with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery1,5
TURALIO is the first FDA-approved targeted therapy for adults with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery1,5
SAFETY



 Find a TGCT Center
Find a TGCT Center















